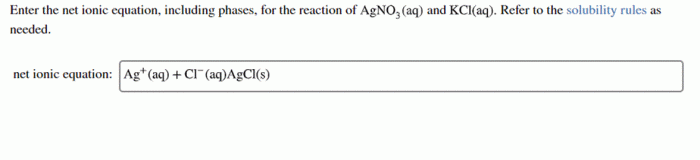Kcl agno3 net ionic equation – The KCl + AgNO3 net ionic equation, a cornerstone of chemistry, unveils the intricate dance of ions and molecules in a captivating precipitation reaction. As potassium chloride (KCl) and silver nitrate (AgNO3) collide, a flurry of ionic interactions ensues, leading to the formation of a dazzling white precipitate: silver chloride (AgCl).
Embark on a journey into the depths of this chemical transformation, where we’ll dissect the net ionic equation, explore its practical applications, and uncover the fascinating world of precipitation reactions.
In this exploration, we’ll delve into the concept of net ionic equations, shedding light on their significance in understanding chemical reactions. We’ll then meticulously dissect the KCl + AgNO3 reaction, tracing the intricate steps of ion exchange and precipitate formation.
Along the way, we’ll unravel the mystery of spectator ions, revealing their role as mere bystanders in the chemical drama.
Definition of Net Ionic Equations
In chemistry, a net ionic equation is a chemical equation that shows only the ions that are actually reacting in a chemical reaction. This type of equation is useful because it allows us to see the essential components of a reaction and to understand the chemical changes that are taking place.
To write a net ionic equation, we start by writing the balanced chemical equation for the reaction. Then, we remove any spectator ions from the equation. Spectator ions are ions that do not participate in the reaction and are present on both sides of the equation.
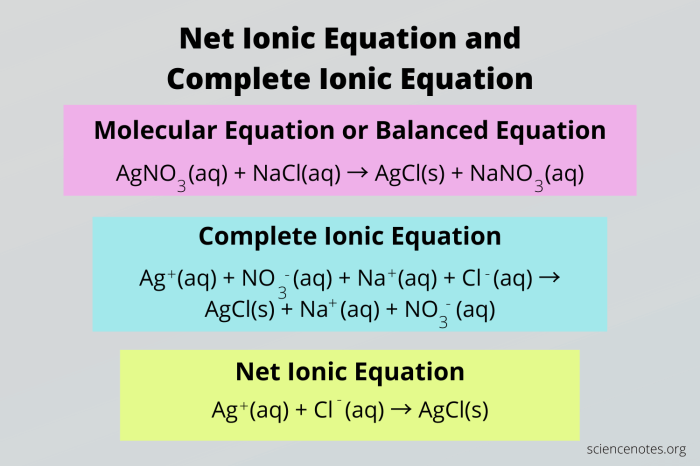
For example, the balanced chemical equation for the reaction between sodium chloride and silver nitrate is:
NaCl + AgNO3→ AgCl + NaNO 3
The spectator ions in this reaction are sodium and nitrate ions. These ions do not participate in the reaction and are present on both sides of the equation. Therefore, the net ionic equation for this reaction is:
Ag++ Cl –→ AgCl
KCL and AgNO3 Reaction
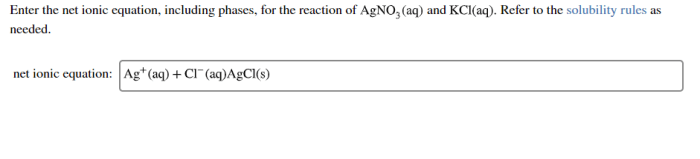
Potassium chloride (KCl) and silver nitrate (AgNO3) are two ionic compounds that react in a double displacement reaction to form silver chloride (AgCl) and potassium nitrate (KNO3).
- The reaction can be represented by the following chemical equation:
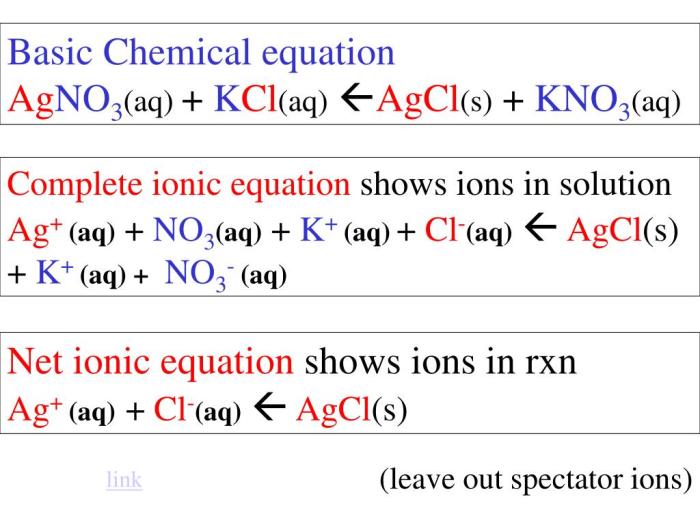
KCl + AgNO3 → AgCl + KNO3
- In this reaction, the potassium ion (K+) from KCl combines with the nitrate ion (NO3-) from AgNO3 to form potassium nitrate (KNO3), while the silver ion (Ag+) from AgNO3 combines with the chloride ion (Cl-) from KCl to form silver chloride (AgCl).
Understanding the net ionic equation for KCl and AgNO3 is crucial for chemistry students. To enhance your knowledge further, you might find the iicrc wrt test answers 2023 resource helpful. Returning to our topic, the net ionic equation for KCl and AgNO3 is a valuable concept in chemistry.
- Silver chloride is a white, insoluble precipitate that forms when the two solutions are mixed.
Writing the Net Ionic Equation
To write the net ionic equation for the reaction between KCl and AgNO3, follow these steps:
1. Write the balanced chemical equation.
- KCl(aq) + AgNO3(aq) → AgCl(s) + KNO3(aq)
2. Identify the spectator ions.
Spectator ions are ions that do not participate in the chemical reaction and remain unchanged throughout the reaction. In this case, K+ and NO3- are the spectator ions.
3. Remove the spectator ions from the equation.
- K+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) → AgCl(s) + K+(aq) + NO3-(aq)
The spectator ions cancel out on both sides of the equation, leaving the net ionic equation:
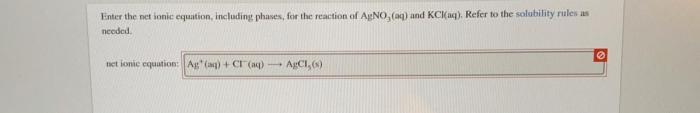
- Ag+(aq) + Cl-(aq) → AgCl(s)
Related Concepts: Kcl Agno3 Net Ionic Equation

Solubility and Net Ionic Equations, Kcl agno3 net ionic equation
Solubility refers to the ability of a substance to dissolve in a solvent. In net ionic equations, only the ions that participate in the reaction are shown. Solubility is related to net ionic equations because it determines which ions are present in the solution and, therefore, which ions can participate in the reaction.
Le Chatelier’s Principle and Precipitation Reactions
Le Chatelier’s principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that counteracts the change. In precipitation reactions, the formation of a solid precipitate shifts the equilibrium to the side of the products, reducing the concentration of the ions in solution.
Essential FAQs
What is a net ionic equation?
A net ionic equation represents a chemical reaction by focusing solely on the ions that undergo a change, excluding spectator ions that do not participate in the reaction.
Why is the KCl + AgNO3 reaction important?
The KCl + AgNO3 reaction is a classic example of a precipitation reaction, where two soluble ionic compounds react to form an insoluble solid precipitate.
How do I write the net ionic equation for the KCl + AgNO3 reaction?
To write the net ionic equation, first identify the spectator ions (K+ and NO3-) and remove them from the equation. The remaining equation, Ag+ + Cl- → AgCl, represents the net ionic equation.